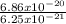Chemistry, 02.09.2019 21:30, graciegirl6662
Water standing in the open at 29.2°c evaporates because of the escape of some of the surface molecules. the heat of vaporization (548 cal/g) is approximately equal to εn, where ε is the average energy of the escaping molecules and n is the number of molecules per gram.
(a) find ε.
(b) what is the ratio of ε to the average kinetic energy of h2o molecules, assuming the latter is related to temperature in the same way as it is for gases?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, shadekashakay
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 02:20, dgadam7495
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 04:30, jocelynmarquillo1
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

Chemistry, 22.06.2019 08:30, ayaanwaseem
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2
Do you know the correct answer?
Water standing in the open at 29.2°c evaporates because of the escape of some of the surface molecul...
Questions in other subjects:



Mathematics, 24.08.2019 10:20

Mathematics, 24.08.2019 10:20



History, 24.08.2019 10:20

Mathematics, 24.08.2019 10:20



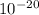 J
J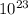 molecules, so n will be:
molecules, so n will be: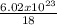
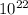 molecules/g
molecules/g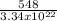
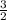 kT
kT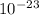 J/K and T = 29.2ºC + 273 = 302.2 K
J/K and T = 29.2ºC + 273 = 302.2 K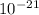 J
J