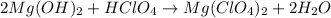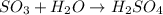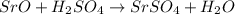Chemistry, 02.09.2019 21:10, jesusmojica25
Complete and balance the equation for the following acid-base neutralization reaction. if water is used as a solvent, write the reactants and products as aqueous ions. in some cases, there may be more than one correct answer, depending on the amount of reactants used.
(a)mg(oh)2 + hclo4?
(b)so3 + h2o? (assume an excess of water and that the product dissolves)
(c) sro + h2so4?

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:20, lindseysmith9522
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1


Chemistry, 23.06.2019 00:20, jessicamcummins
What type of context clue you understand the meaning of quandary?
Answers: 3
Do you know the correct answer?
Complete and balance the equation for the following acid-base neutralization reaction. if water is u...
Questions in other subjects:


Mathematics, 21.01.2020 08:31


Geography, 21.01.2020 08:31




English, 21.01.2020 08:31


Chemistry, 21.01.2020 08:31


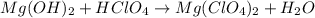
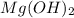 by 2 on reactant side and multiply
by 2 on reactant side and multiply 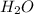 by 2 on product side.
by 2 on product side.