
Chemistry, 02.09.2019 21:10, lekaje2375
Identify the atoms that are oxidized and reduced, the change in the oxidation state for each, and oxidising and reducing agents in each of the following equations:
(a)mg + nicl2 -> mgcl2 + ni
(b)pcl3 + cl2 -> pcl5
(c)c2h4 + 3o2 -> 2co2 + 2h2o
(d)zn + h2so4 -> h2 + znso4
(e)2k2s2o3 + i2- > k2s4o6+2ki
(f)3cu + 8hno3 = 3cu(no3)2 + 4h2o + 2no

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, amandasantiago2001
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

Chemistry, 22.06.2019 13:30, bryce99
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 22.06.2019 14:30, Hannahmiller3773
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3
Do you know the correct answer?
Identify the atoms that are oxidized and reduced, the change in the oxidation state for each, and ox...
Questions in other subjects:

Mathematics, 16.10.2020 22:01

Mathematics, 16.10.2020 22:01

Social Studies, 16.10.2020 22:01



History, 16.10.2020 22:01




History, 16.10.2020 22:01


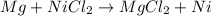
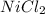 acts as oxidizing agent.
acts as oxidizing agent.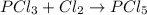
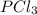 acts as reducing agent and
acts as reducing agent and 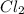 acts as oxidizing agent.
acts as oxidizing agent.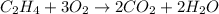
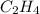 acts as reducing agent and
acts as reducing agent and 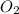 acts as oxidizing agent.
acts as oxidizing agent.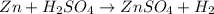
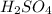 acts as oxidizing agent
acts as oxidizing agent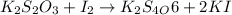
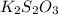 acts as reducing agent and
acts as reducing agent and 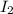 acts as oxidizing agent.
acts as oxidizing agent.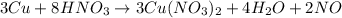
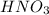 acts as oxidizing agent
acts as oxidizing agent



