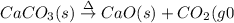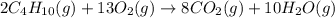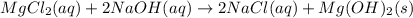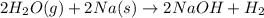Write a balanced molecular equation describing each of the following chemical reactions:
(a)s...

Chemistry, 02.09.2019 20:10, codyshs160
Write a balanced molecular equation describing each of the following chemical reactions:
(a)solid calcium carbonate is heated and decomposes to sold calcium oxide and carbon dioxide
(b)gaseous butane, c4h10, reacts with diatomic oxygen gas to yield gaseous carbon dioxide and water vapor
(c)aaqeous solutions of magnesium chloride and sodium hydroxide react to produce solid magnesium hydroxide and aqueous sodium chloride
(d)water vapor reacts with sodium metal to produce solid sodium hydroxide and hydrogen gas

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, NREYESLDS2806
To save time, you can approximate the initial mass of the solid to the nearest ±1 g. for example, if you are asked to add 14.3 g of copper, add between 13 g and 15 g. which of the following sets include two samples with an equal density? which all that apply below 15.4 g gold and 18.7 g silver 15.2 g copper and 50.0 g copper 20.2 g silver and 20.2 g copper 11.2 g gold and 14.9 g gold
Answers: 1

Chemistry, 22.06.2019 11:00, 21villalobosjabez
Which type of fossil does this image depict?
Answers: 1

Chemistry, 22.06.2019 18:10, ellemarshall13
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Mathematics, 26.10.2021 16:40

History, 26.10.2021 16:40




History, 26.10.2021 16:40




Physics, 26.10.2021 16:40