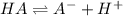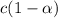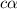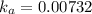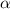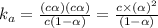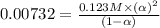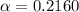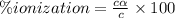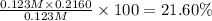Chemistry, 02.09.2019 19:20, daniel1480
the ka of a monoprotic weak acid is 0.00732. what is the percent ionization of a 0.123 m solution of this acid?
percent ionization: %

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 19:30, Adrian12313
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1

Chemistry, 23.06.2019 00:30, vane6176
You are attempting to recrystallize a crude product mixture. you add the appropriate amount of hot solvent and are allowing the solution to slowly cool to room temperature. however, at room temperature no crystals have appeared, which of the following methods should be used to induce crystallization? choose all correct answers. a) place the flask in an ice bath. b) swirl the contents of the flask. c) add a small seed crystal of the desired product. d) scratch the inside of the glassware using a stir rod. it can be multiple choices
Answers: 3

Chemistry, 23.06.2019 00:30, clairebear66
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1
Do you know the correct answer?
the ka of a monoprotic weak acid is 0.00732. what is the percent ionization of a 0.123 m solution of...
Questions in other subjects:

Mathematics, 15.07.2020 15:01


Mathematics, 15.07.2020 15:01



English, 15.07.2020 15:01

Social Studies, 15.07.2020 15:01