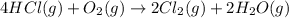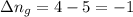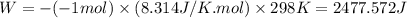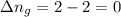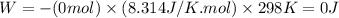Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:40, timmonskids6027
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 06:00, palomaresmitchelle
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1


Chemistry, 22.06.2019 22:30, lanashanabJHsbd1099
Who discovered a pattern to the elements in 1869?
Answers: 1
Do you know the correct answer?
11. assuming that the gases are ideal, calculate the amount of work done (joules) in each of the fol...
Questions in other subjects:


Chemistry, 26.06.2021 04:30

Mathematics, 26.06.2021 04:30

History, 26.06.2021 04:30

Mathematics, 26.06.2021 04:30






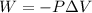
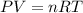
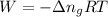 ......(1)
......(1)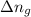 = difference in number of moles of products and reactants =
= difference in number of moles of products and reactants = 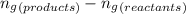
![25^oC=[273+25]K=298K](/tpl/images/0221/2455/0e82f.png)