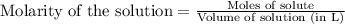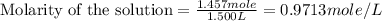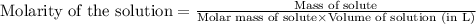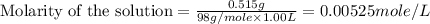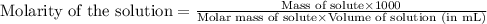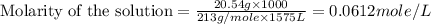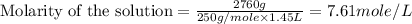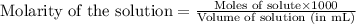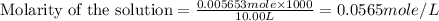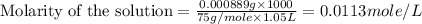Chemistry, 02.09.2019 18:20, aubreyfoster
Determine the molarity for each of the following solution solutions:
(a)1.457 mol of kcl in 1.500 l of solution
(b) 0.515 gram ofh2so4, in 1.00 l of solution
(c) 20.54 g of al(no3)3 in 1575 ml of solution
(d)2.76 kg ofcuso4.5h2o in 1.45 l of solution
(e)0.005653 mol ofbr2 in 10.00 ml of solution
(f) 0.000889 g of glycine, c2h5no2, in 1.05 ml of solution

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, erikloza12pdidtx
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1

Chemistry, 22.06.2019 03:00, annafellows
Match term definition ellipse a) diagonal cross section of a cylinder circle b) diagonal cross section through the widest part of a sphere sphere c) cross section parallel to the base of a cone great circle d) shape created when a semi-circle is rotated around the y-axis triangle e) perpendicular cross section of a cone
Answers: 1

Chemistry, 22.06.2019 19:40, jholland03
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1
Do you know the correct answer?
Determine the molarity for each of the following solution solutions:
(a)1.457 mol of kcl in...
(a)1.457 mol of kcl in...
Questions in other subjects:









Chemistry, 26.02.2020 23:27


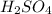 solution is, 0.00525 mole/L
solution is, 0.00525 mole/L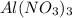 solution is, 0.0612 mole/L
solution is, 0.0612 mole/L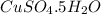 solution is, 7.61 mole/L
solution is, 7.61 mole/L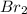 solution is, 0.0565 mole/L
solution is, 0.0565 mole/L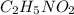 solution is, 0.0113 mole/L
solution is, 0.0113 mole/L