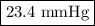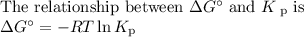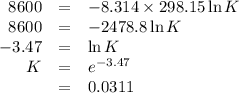Chemistry, 31.08.2019 03:10, arianna2814
Consider the reaction at 25 °c. h2o(l) ↔ h2o(g) δg° = 8.6 kj/mol calculate the pressure of water at 25 °c (hint: get k eq)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, kkmonsterhigh18
The diagram below shows a cell placed in a solution. a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution. only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it. it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2

Chemistry, 22.06.2019 11:30, ayoismeisjjjjuan
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 20:30, huangjianhe135
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3

Chemistry, 22.06.2019 22:30, eduardoguizar8787
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1
Do you know the correct answer?
Consider the reaction at 25 °c. h2o(l) ↔ h2o(g) δg° = 8.6 kj/mol calculate the pressure of water at...
Questions in other subjects:


History, 10.06.2020 02:57






Chemistry, 10.06.2020 02:57

Mathematics, 10.06.2020 02:57

History, 10.06.2020 02:57