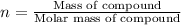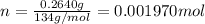Chemistry, 31.08.2019 02:30, pearpeaerrr1993
3.0.2640 g of sodium oxalate is dissolved in a flask and requires 30.74 ml of potassium permanganate to titrate it to turn pink end point. the equation for this reaction is: 5na, c,o4+ 2kmno,+ 8h, so 2mnsog+ k, so,+ 5 na, so,+ 10co2+ 8h2o a) how many moles of sodium oxalate are present in the flask?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:40, carebear60
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 22.06.2019 20:30, trevorhenyan51
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1


Chemistry, 23.06.2019 04:10, nabeelunique
An unknown substance has been shown to have metallic bonds. which of the following is most likely a property of this substance? a. low conductivity b. low boiling point c. high malleability d. high solubility in water
Answers: 2
Do you know the correct answer?
3.0.2640 g of sodium oxalate is dissolved in a flask and requires 30.74 ml of potassium permanganate...
Questions in other subjects:

Mathematics, 04.11.2020 05:00


Chemistry, 04.11.2020 05:00








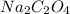 = 134 g/mol
= 134 g/mol