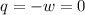Asample consisting of 2 moles he is expanded isothermally at 0 degrees from 5.0dm3 to 20.0dm3. calculate w, q and deltau for each of the following situations: (i) a reversible expansion of the sample. (ii) an irreversible expansion of the sample against a constant external pressure equal to the final pressure of the gas. (iii) a free expansion (against zero external pressure i. e. in a vacuum) of the sample.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, stelllllllllllllllla
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 11:40, Wemaybewrong
Modern pennies are composed of zinc coated with copper. a student determines the mass of a penny to be 2.482 g and then makes several scratches in the copper coaling (to expose the underlying zinc). the student puts the scratched penny in hydrochloric acid, where the following reaction occurs between the zinc and the hcl (the copper remains undissolved): zn(s) + 2 hcl(aq) → h2(g) + zncl(aq)the student collects the hydrogen produced over water at 25 °c. the collected gas occupies a volume of 0.899 l at a total pressure of 79 j mmhg. calculate the percent zinc (by mass) in the penny. (assume that all the zn in the penny dissolves.)
Answers: 1

Chemistry, 22.06.2019 14:30, CoolRahim9090
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1

Chemistry, 22.06.2019 16:40, roderickhinton
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3
Do you know the correct answer?
Asample consisting of 2 moles he is expanded isothermally at 0 degrees from 5.0dm3 to 20.0dm3. calcu...
Questions in other subjects:


Mathematics, 04.11.2020 18:20

English, 04.11.2020 18:20




English, 04.11.2020 18:20



Social Studies, 04.11.2020 18:20


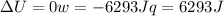
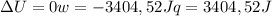
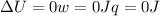
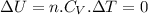 since
since 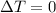
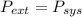 so
so 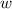 can be calculated by
can be calculated by 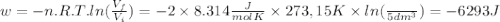
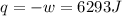
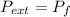
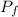 by the law of ideal gases
by the law of ideal gases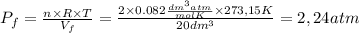
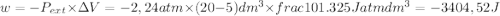
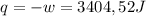
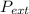 so
so 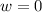 (there's no work at vaccum) and
(there's no work at vaccum) and