
Calculate the amount of heat energy in kj required to convert 45.0 g of ice at -15.5'c to steam at 124.0°c. (cwater 118 jig'c, gee 2.03 jig c, g team jig c, molar heat of fusion of ice 6.01 * 10 j/mol; molar heat of vaporization of liquid water 4.07 * 10*j/mol 202 short answer toolbar navigation b i v s e 1 e a a this question will be sent to your instructor for grading

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 22:10, steven0448
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1

Chemistry, 23.06.2019 03:00, sharondacarruth1656
Is it safe to take 450mg of diphenhydramine hydrochloride?
Answers: 1
Do you know the correct answer?
Calculate the amount of heat energy in kj required to convert 45.0 g of ice at -15.5'c to steam at 1...
Questions in other subjects:


Business, 24.10.2020 23:10


Mathematics, 24.10.2020 23:10

English, 24.10.2020 23:10



English, 24.10.2020 23:10

Mathematics, 24.10.2020 23:10


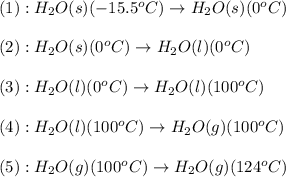
![\Delta H=[m\times c_{p,s}\times (T_{final}-T_{initial})]+n\times \Delta H_{fusion}+[m\times c_{p,l}\times (T_{final}-T_{initial})]+n\times \Delta H_{vap}+[m\times c_{p,g}\times (T_{final}-T_{initial})]](/tpl/images/0213/6323/e4ef0.png)
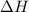 = enthalpy change or heat required = ?
= enthalpy change or heat required = ?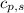 = specific heat of solid water =
= specific heat of solid water = 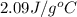
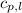 = specific heat of liquid water =
= specific heat of liquid water = 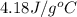
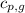 = specific heat of liquid water =
= specific heat of liquid water = 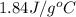
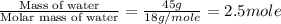
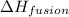 = enthalpy change for fusion = 6.01 KJ/mole = 6010 J/mole
= enthalpy change for fusion = 6.01 KJ/mole = 6010 J/mole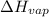 = enthalpy change for vaporization = 40.67 KJ/mole = 40670 J/mole
= enthalpy change for vaporization = 40.67 KJ/mole = 40670 J/mole![\Delta H=[45g\times 4.18J/gK\times (0-(-15.5))^oC]+2.5mole\times 6010J/mole+[45g\times 2.09J/gK\times (100-0)^oC]+2.5mole\times 40670J/mole+[45g\times 1.84J/gK\times (124-100)^oC]](/tpl/images/0213/6323/555ef.png)
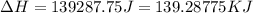 (1 KJ = 1000 J)
(1 KJ = 1000 J)



