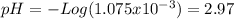Chemistry, 30.08.2019 23:30, apolloplays10
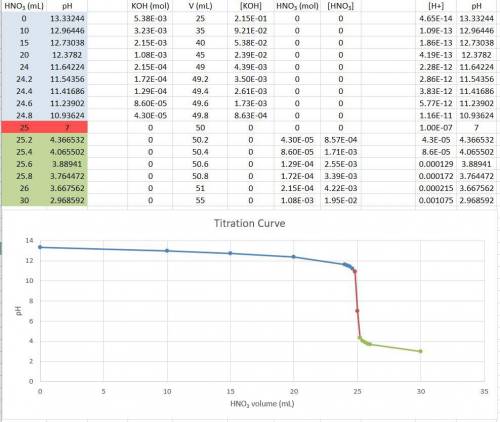
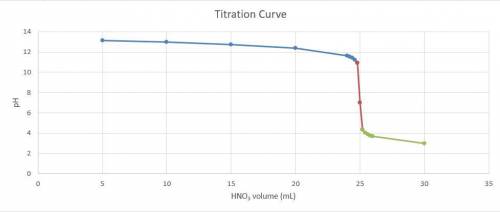
Consider the titration of a 25 ml sample of 0.215 m koh is titrated with 0.215 m hno3 a. calculate the volume of added acid that is required to reach equivalence point. (hint: what equals what at the equivalence point? ) b. the initial ph of koh c. calculate the ph after adding 5.0 ml of hno,. (hint: what kind of solution have you made? what equation can you use to calculate the ph? ) d. the ph after adding 10.0, 15.0, 20.0 ml e. the ph at the equivalence point f. the ph after adding 30 ml of hno g. sketch the titration curve. include labels for equivalence point, where there is excess oh and where there is excess h, o*.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:20, emilyborland50
Which of the following statements is false regarding aromaticity? a. the compound must be cyclic b. the compound must be fully conjugated c. the compound must be planar d. the number of electrons in the pi system must satisfy the hückel 4n+2 rule e. the compound must have a neutral charge
Answers: 2


Do you know the correct answer?
Consider the titration of a 25 ml sample of 0.215 m koh is titrated with 0.215 m hno3 a. calculate t...
Questions in other subjects:

Biology, 28.06.2019 21:20

Biology, 28.06.2019 21:20


Biology, 28.06.2019 21:20



Biology, 28.06.2019 21:20

Biology, 28.06.2019 21:20


Biology, 28.06.2019 21:20


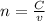
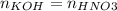
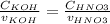
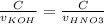
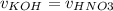
![[OH^{-} ]=\frac{moles of KOH - moles of HNO3}{total volume}](/tpl/images/0213/3384/4dd62.png)
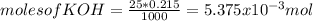
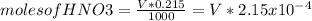
![[OH^{-} ]=\frac{5.375x10^{-3}- V*2.15x10^{-4}}{(\frac{25+v}{1000} )}](/tpl/images/0213/3384/e913a.png)
![[OH^{-} ]=\frac{5.375x10^{-3}- 5*2.15x10^{-4}}{(\frac{25+5}{1000} )}=0.143](/tpl/images/0213/3384/79893.png)
![[OH^{-} ]=\frac{5.375x10^{-3}- 10*2.15x10^{-4}}{(\frac{25+10}{1000} )}=0.0921](/tpl/images/0213/3384/e9b6e.png)
![[OH^{-} ]=\frac{5.375x10^{-3}- 15*2.15x10^{-4}}{(\frac{25+15}{1000} )}=0.0538](/tpl/images/0213/3384/ef1fb.png)
![[OH^{-} ]=\frac{5.375x10^{-3}- 20*2.15x10^{-4}}{(\frac{25+20}{1000} )}=0.0239](/tpl/images/0213/3384/6aacc.png)
![[H^{+} ]=\frac{(V-25)*2.15x10^{-4}}{\frac{V+25}{1000} }](/tpl/images/0213/3384/54d05.png)
![[H^{+} ]=\frac{(30-25)*2.15x10^{-4}}{\frac{30+25}{1000} } =1.075x10^{-3}](/tpl/images/0213/3384/583ea.png)