Hydrazine, n2h4, is a weak base and is used as fuel in the space shuttle.
n2h4(aq)+h2o(l)ân2h5...


Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 01:30, lizethdominguez037
What is the value of keq for the reaction expressed in scientific notation
Answers: 1

Chemistry, 22.06.2019 08:30, melikefood01
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 10:40, rntaran2002
What is the ph of a 0.0010 m hno3? 1.0 3.0 4.0 5.0
Answers: 2
Do you know the correct answer?
Questions in other subjects:

Mathematics, 10.12.2020 01:30



Mathematics, 10.12.2020 01:30


Mathematics, 10.12.2020 01:30



Health, 10.12.2020 01:30


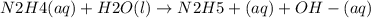
![Kb = \frac{[N2H5+][OH-]}{[N2H4]}------(1)](/tpl/images/0213/3718/63a47.png)
![[OH-] = 10^{-pOH} =10^{-3.34} =4.57*10^{-4} M](/tpl/images/0213/3718/5c095.png)
![Kb = \frac{[4.57*10^{-4}]^{2}}{[0.133]}=1.6*10^{-6}](/tpl/images/0213/3718/3b5bb.png)




