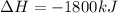Manganese metal can be obtained by the reaction of manganese dioxide with aluminium. 4al(s) + 3mnoz(s) → 2 al2o3(s) + 3 mn(s) calculate the enthalpy change of reaction. given: 2al(s) + 3/2 o2(g) →al2o3(s) ah = -1680 kj mol'. mn(s) + o2(g) → mno2(s) ah = -520 kj mol! .

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, aubreykenzie686
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1

Chemistry, 21.06.2019 23:30, hellokitty1647
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i. e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 05:30, palcochran1313
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1
Do you know the correct answer?
Manganese metal can be obtained by the reaction of manganese dioxide with aluminium. 4al(s) + 3mnoz(...
Questions in other subjects:




Mathematics, 14.02.2020 01:45


Biology, 14.02.2020 01:45




Mathematics, 14.02.2020 01:45


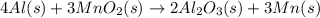
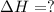
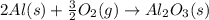
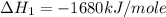
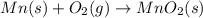
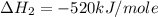
![\Delta H=[n\times \Delta H_1]+[n\times (-\Delta H_2)]](/tpl/images/0213/0280/c45d3.png)
![\Delta H=[2mole\times (-1680kJ/mole)]+[3\times -(-520kJ/mole)]](/tpl/images/0213/0280/514b6.png)