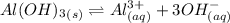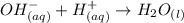Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:40, timmonskids6027
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1


Chemistry, 22.06.2019 21:00, melissalopez12
Acandle’s wick is the fabric string that holds the flame, and it burns down at a constant slow pace when the candle is lit. the wick is usually surrounded by wax. which is the most important property of covalent compounds that makes them useful for making candle wax? a low boiling point a low melting point a high boiling point a high melting point
Answers: 1

Chemistry, 22.06.2019 23:30, Xavier8247
Rank the following four acids in order of increasing bronsted acidity : h2f+ , ch3oh, (ch3)2oh+ , ch3sh2+
Answers: 3
Do you know the correct answer?
Consider the equilibrium of al(oh)3 (s) in water. al(oh)3 (s) = a13+ (aq) + 30h" (aq) how is the sol...
Questions in other subjects:

Computers and Technology, 26.07.2019 22:30

Mathematics, 26.07.2019 22:30

Physics, 26.07.2019 22:30

Health, 26.07.2019 22:30


Physics, 26.07.2019 22:30