
Chemistry, 30.08.2019 17:30, Reggie101127
Be sure to answer all parts. what is the [h3o+] and the ph of a buffer that consists of 0.26 m hno2 and 0.89 m kno2? (k, of hno2 = 7.1 x 10-4) fh 0+1= x 10 m (enter your answer in scientific notation.) ph =

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 23.06.2019 00:50, maddysmall32
Which of the following warnings would an agricultural chemist tell a farmer who wants to recycle his or her own ammonia? recycling ammonia is a difficult process that sometimes takes weeks. recycling ammonia requires a degree in biochemistry or a related field. recycling ammonia can be harmful because it is highly flammable and toxic. recycling ammonia costs too much money considering the price of the necessary chemicals.
Answers: 1
Do you know the correct answer?
Be sure to answer all parts. what is the [h3o+] and the ph of a buffer that consists of 0.26 m hno2...
Questions in other subjects:

Mathematics, 22.11.2020 18:40

Biology, 22.11.2020 18:40

History, 22.11.2020 18:40





History, 22.11.2020 18:40


Chemistry, 22.11.2020 18:40


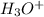 ion concentration is,
ion concentration is, 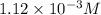 and the pH of a buffer is, 2.95
and the pH of a buffer is, 2.95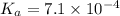
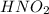 (weak acid)= 0.26 M
(weak acid)= 0.26 M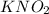 (conjugate base or salt)= 0.89 M
(conjugate base or salt)= 0.89 M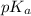 .
.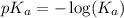
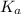 in this expression, we get:
in this expression, we get: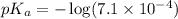
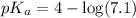
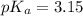
![pH=pK_a+\log \frac{[Salt]}{[Acid]}](/tpl/images/0212/5080/e961a.png)
![pH=pK_a+\log \frac{[KNO_2]}{[HNO_2]}](/tpl/images/0212/5080/600d6.png)
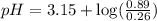
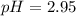
![pH=-\log [H_3O^+]](/tpl/images/0212/5080/841e8.png)
![2.95=-\log [H_3O^+]](/tpl/images/0212/5080/3df02.png)
![[H_3O^+]=1.12\times 10^{-3}M](/tpl/images/0212/5080/0bf52.png)




