
Chemistry, 30.08.2019 17:20, carrietaylor234
For the following equilibrium: 3a + b = 20 if equilibrium concentrations are [a] = 1.1 m and [b] = 1.4 m, and kc = 11.3, what is the equilibrium concentration of c? • your answer should have two significant figures. provide your answer below:

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, kichensides
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Chemistry, 22.06.2019 18:30, madmatt873
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1

Chemistry, 22.06.2019 22:00, robert7248
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1

Chemistry, 22.06.2019 23:00, Mynameismath
Which type of intermolecular attractions holds ammonia molecules together with other ammonia molecules?
Answers: 3
Do you know the correct answer?
For the following equilibrium: 3a + b = 20 if equilibrium concentrations are [a] = 1.1 m and [b] =...
Questions in other subjects:



Mathematics, 13.10.2020 21:01

Physics, 13.10.2020 21:01

Mathematics, 13.10.2020 21:01

Health, 13.10.2020 21:01

Mathematics, 13.10.2020 21:01

Mathematics, 13.10.2020 21:01


English, 13.10.2020 21:01


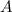 at equilibrium = 1.1 M
at equilibrium = 1.1 M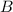 at equilibrium = 1.4 M
at equilibrium = 1.4 M
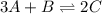
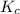 will be,
will be,![K_c=\frac{[C]^2}{[A]^3[B]}](/tpl/images/0212/4597/0cfd6.png)
![11.3=\frac{[C]^2}{(1.1)^3\times (1.4)}](/tpl/images/0212/4597/67486.png)
![[C]=4.6M](/tpl/images/0212/4597/2aa7c.png)




