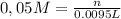Alaboratory scientist wants to analyze the chlorogenic acid content of a 0.5 l sample. he uses an indicator solution and titrates with sodium hydroxide. he finds the equivalence point occurs when he's titrated 9.5 ml of 0.05 m sodium hydroxide. approximately how much chlorogenic acid is in the sample? 475 umol ® 480 umol © 500 umol 510 umol

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:40, wanderer3653
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 06:00, kylieweeks052704
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

Chemistry, 22.06.2019 21:00, nsutton9985
Two nails have identical sizes and shapes. in one nail, 20 percent of the domains are lined up. in the other nail, 80 percent of the domains are lined up. which has stronger magnetic force? first answer gets brainliest!
Answers: 1

Chemistry, 23.06.2019 00:30, lareynademividp0a99r
Gasoline has a density of 0.740 g/ml. if you have 328 grams of gasoline, what is the volume in milliliters?
Answers: 1
Do you know the correct answer?
Alaboratory scientist wants to analyze the chlorogenic acid content of a 0.5 l sample. he uses an in...
Questions in other subjects:






Physics, 04.04.2020 04:15


Mathematics, 04.04.2020 04:15

Mathematics, 04.04.2020 04:15