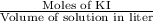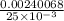Chemistry, 30.08.2019 05:30, xxaurorabluexx
The concentration of a potassium iodide solution can be determined by first adding excess silver nitrate: i'(aq) + ag+ (aq) → agl(s) the excess silver ion remaining in solution is then determined by reaction with a potassium thiocyanate (kscn) solution of known concentration: ag (aq) + scn(aq) → agscn(s) in an experiment, 50.00 ml of 0.0565 m agno3 was added to 25.00 ml of a potassium iodide solution. it then took 8.32 ml of 0.0510 m kscn solution to precipitate the unreacted silver ions. what is the concentration of the original ki solution?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, SilverTheAmarok
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 2

Chemistry, 22.06.2019 19:30, liyahlanderson2232
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2

Chemistry, 23.06.2019 04:31, woodfordmaliky
One student said that the investigation was not valid (a fair test). write a plan for the investigation that includes improvements to the method and apparatus
Answers: 1
Do you know the correct answer?
The concentration of a potassium iodide solution can be determined by first adding excess silver nit...
Questions in other subjects:

Mathematics, 25.01.2021 14:00

Mathematics, 25.01.2021 14:00

Biology, 25.01.2021 14:00


Mathematics, 25.01.2021 14:00



Mathematics, 25.01.2021 14:00

Biology, 25.01.2021 14:00

Physics, 25.01.2021 14:00