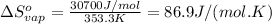Chemistry, 30.08.2019 01:30, veneciaconton347
The enthalpy of vaporization (δh°vap) of benzene is 30.7 kj/mol at its normal boiling point of 353.3 k. what is δs°vap at this temperature? a. 383 j/(mol·k) b. 0.0115 j/(mol·k) c. 86.9 j/(mol·k) d. 0.087 j/(mol·k) e. 11.5 j/(mol·k)

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:30, justinerodriguz2878
What are the major types of a chemical compound
Answers: 2

Chemistry, 22.06.2019 14:00, rosetoheart2
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2
Do you know the correct answer?
The enthalpy of vaporization (δh°vap) of benzene is 30.7 kj/mol at its normal boiling point of 353.3...
Questions in other subjects:



Mathematics, 16.12.2019 22:31



English, 16.12.2019 22:31

Mathematics, 16.12.2019 22:31

Biology, 16.12.2019 22:31

Mathematics, 16.12.2019 22:31

History, 16.12.2019 22:31


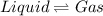
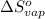 for the reaction, we use the equation:
for the reaction, we use the equation: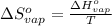
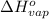 = standard enthalpy change of vaporization = 30.7 kJ/mol = 30700 J/mol (Conversion factor: 1 kJ = 1000 J)
= standard enthalpy change of vaporization = 30.7 kJ/mol = 30700 J/mol (Conversion factor: 1 kJ = 1000 J)