
Chemistry, 29.08.2019 20:20, jessnolonger
Carbon, hydrogen and ethane each burn exothermically in an excess of air. ahⓡ =-393.7 kj mol. c(s) + o2(g) → co2(g) h2(g) + % o2(g) → h20(1) czha(g) + 302() → 2co2(g) + 2h2o(1) ah®=-285.9 kj mol ah =-1411.0 kj moll. use the data to calculate the standard enthalpy change of formation, ah in kj mol'', of ethene at 298 k 2c(s) + 2h2(g) → c2h4(g)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, officialalex6330
Balance and in which category does it fit in? single or double displacement or synthesis or decomposition? (a) k2 o → k + o2 (b) na + i2 → nai (c) cu(no3 )2 + naoh → cu(oh)2 + nano3 (d) kclo3 → kcl + o2 (e) ca(no3 )2 + hbr → cabr2 + hno3 (f) sn(oh)2 → sno + h2 o (g) p4 + n2 o → p4 o6 + n2 (h) fe + al2 (so4 )3 → feso4 + al (i) alcl3 + na2 co3 → al2 (co3 )3 + nacl (j) c3 h6 + o2 → co2 + h2 o
Answers: 1

Chemistry, 22.06.2019 11:30, samantha9430
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 21:30, leenzazou587
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1
Do you know the correct answer?
Carbon, hydrogen and ethane each burn exothermically in an excess of air. ahⓡ =-393.7 kj mol. c(s) +...
Questions in other subjects:

Mathematics, 21.05.2020 22:00

Mathematics, 21.05.2020 22:00


Mathematics, 21.05.2020 22:00

Chemistry, 21.05.2020 22:00






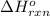 for the reaction is 51.8 kJ.
for the reaction is 51.8 kJ.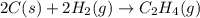
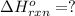
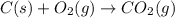
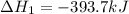 ( × 2)
( × 2)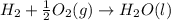
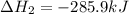 ( × 2)
( × 2)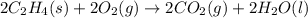

![\Delta H^o_{rxn}=[2\times \Delta H_1]+[2\times \Delta H_2]+[1\times (-\Delta H_3)]](/tpl/images/0209/7515/e45ac.png)
![\Delta H^o_{rxn}=[(2\times (-393.7))+(2\times (-285.9))+(1\times -(-1411))]=51.8kJ](/tpl/images/0209/7515/5c6c6.png)





