
Chemistry, 29.08.2019 18:20, lilyella1004
The central atom in the iodite ion, 102, is surrounded by 1 [2] [3] [4] [5] single bond, i double bond and 2 lone pairs of electrons. 3 bonding pairs and i unshared pair of electrons. 1 bonding pair and 3 unshared pairs of electrons. 2 double bonds and no unshared pairs of electrons. 4 bonding pairs and 4 lone pairs of electrons.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:40, jerrysandoval22
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2


Chemistry, 22.06.2019 22:40, lindseyklewis1p56uvi
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization. a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution. part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1
Do you know the correct answer?
The central atom in the iodite ion, 102, is surrounded by 1 [2] [3] [4] [5] single bond, i double bo...
Questions in other subjects:

Mathematics, 19.05.2020 20:13


Mathematics, 19.05.2020 20:13

Mathematics, 19.05.2020 20:13



Biology, 19.05.2020 20:14



Biology, 19.05.2020 20:14


![\text{Number of electrons}=\frac{1}{2}[V+N-C+A]](/tpl/images/0209/4471/86ecf.png)
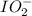 molecules.
molecules.![\text{Number of electrons}=\frac{1}{2}\times [7+1]=4](/tpl/images/0209/4471/1d68f.png)
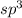 and the electronic geometry of the molecule will be tetrahedral.
and the electronic geometry of the molecule will be tetrahedral.![The central atom in the iodite ion, 102, is surrounded by 1 [2] [3] [4] [5] single bond, i double bo](/tpl/images/0209/4471/6d8db.jpg)




