
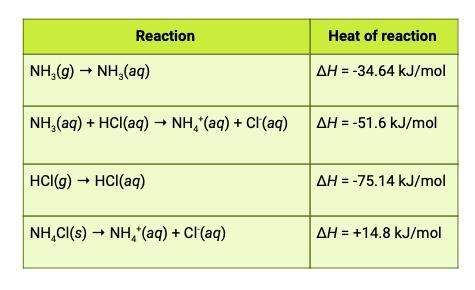
The heat of reaction for the process described in (a) can be determined by applying hess's law. the heats of reaction shown in the table below can be obtained experimentally or looked up in tables of enthalpy data. which two of these heats of reaction would be the easiest and safest to measure in the laboratory, and which two are better obtained through reference sources? why? hint: consider whether a reaction takes place in aqueous solution or instead involves noxious gases.
this is the equation for a: δh25° = ( δh25° nh3 + δh25° hcl) - ( δh25° nh4cl)
and attached is the table

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 22:30, mimireds5419
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3

Chemistry, 22.06.2019 06:20, stephliu721
What is a property of a double replacement reaction
Answers: 1

Chemistry, 22.06.2019 11:00, peternice2956
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1
Do you know the correct answer?
The heat of reaction for the process described in (a) can be determined by applying hess's law. the...
Questions in other subjects:

English, 14.05.2021 01:00

Mathematics, 14.05.2021 01:00



Chemistry, 14.05.2021 01:00




Social Studies, 14.05.2021 01:00






