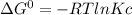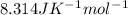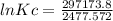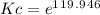Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, robert7248
What is the cellular process that releases the energy stored in food molecules
Answers: 3

Chemistry, 22.06.2019 04:50, psychocatgirl1
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 09:00, kcarstensen59070
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1
Do you know the correct answer?
Calculate the equilibrium constant kc at 25 c from the free - energy change for the following reacti...
Questions in other subjects:





Business, 23.11.2020 19:40

Mathematics, 23.11.2020 19:40


Mathematics, 23.11.2020 19:40

Mathematics, 23.11.2020 19:40

Health, 23.11.2020 19:40


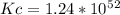
![Kc=\frac{[Zn^+^2]}{[Ag^+]^2}](/tpl/images/0207/5568/de926.png)
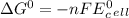
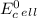 can be calculated using standard reduction potentials.
can be calculated using standard reduction potentials.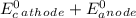
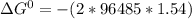
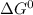 = -297173.8 J
= -297173.8 J