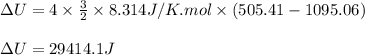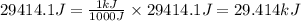Chemistry, 28.08.2019 23:00, michaellangley
Acompression, at a constant pressure of 140 kpa, is performed on 4.0 moles of an ideal monatomic gas (cv = 3/2 r). the compression reduces the volume of the gas from 0.26 m^3 to 0.12 m^3. the change in the internal energy of the gas, in kj is ("^3" means to the power of 3)

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 15:30, 20cschultz
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 22.06.2019 16:30, joshua1255
Find the number of moles of argon in 364g of argon.
Answers: 2

Chemistry, 22.06.2019 16:50, Pookiev
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1
Do you know the correct answer?
Acompression, at a constant pressure of 140 kpa, is performed on 4.0 moles of an ideal monatomic gas...
Questions in other subjects:


Mathematics, 04.03.2021 20:40


World Languages, 04.03.2021 20:40



Social Studies, 04.03.2021 20:40

Mathematics, 04.03.2021 20:40



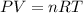
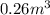
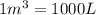
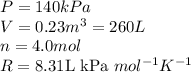
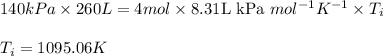
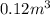
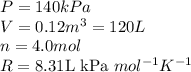
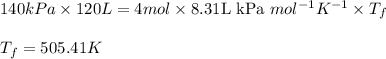
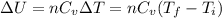
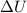 = change in internal energy = ?
= change in internal energy = ?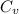 = heat capacity at constant volume =
= heat capacity at constant volume = 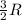
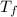 = final temperature = 1095.06 K
= final temperature = 1095.06 K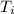 = initial temperature = 505.41 K
= initial temperature = 505.41 K