
For the reaction mgso3(s) → mgo(s) + so2(g), which is spontaneous only at high temperatures, one would predict thata. δh˚ is negative and δs˚ is negativeb. δh˚ is positive and b. δs˚ is negativec. δh˚ is positive and δs˚ is positived. δh˚ is negative and δs˚ is positive

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, natalie857123
For each of the following types of reactions, write a general reaction formula in the symbolic form—for example, a + b → ab. single-displacement double-displacement synthesis decomposition
Answers: 1

Chemistry, 22.06.2019 02:30, brittanysanders
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 10:30, tjjjjjjjjjjjjjjjjjjj
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 17:20, holmesleauja
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2
Do you know the correct answer?
For the reaction mgso3(s) → mgo(s) + so2(g), which is spontaneous only at high temperatures, one wou...
Questions in other subjects:

French, 13.12.2021 15:10


Mathematics, 13.12.2021 15:10

Business, 13.12.2021 15:10


History, 13.12.2021 15:10

History, 13.12.2021 15:10

Chemistry, 13.12.2021 15:10


English, 13.12.2021 15:10


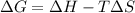
 = Gibbs free energy
= Gibbs free energy  = enthalpy change
= enthalpy change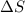 = entropy change
= entropy change 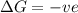
 >
> 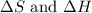 both have positive values.
both have positive values. 





