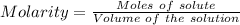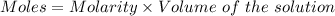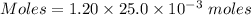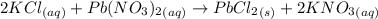Chemistry, 28.08.2019 17:30, leomessifanboy678
A25.0-ml sample of a 1.20 m potassium chloride solution is mixed with 15.0 ml of a 0.900 m lead(ii) nitrate solution and this precipitation reaction occurs: 2 kcl(aq)+pb(no3)2(aq)→pbcl2(s)+2 kno3(aq) the solid pbcl2 is collected, dried, and found to have a mass of 2.45 g. determine the limiting reactant, the theoretical yield, and the percent yield.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, only1cache
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 16:00, rorymartin04
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2

Chemistry, 22.06.2019 20:40, oddoneshenchman
Why do lunar and solar eclipse not happen every month
Answers: 2
Do you know the correct answer?
A25.0-ml sample of a 1.20 m potassium chloride solution is mixed with 15.0 ml of a 0.900 m lead(ii)...
Questions in other subjects:

Mathematics, 23.07.2020 01:01

Mathematics, 23.07.2020 01:01




Computers and Technology, 23.07.2020 01:01

Physics, 23.07.2020 01:01