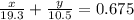Consider a piece of gold jewelry that weighs 9.85 g and has a volume of 0.675 cm3 . the jewelry contains only gold and silver, which have densities of 19.3 g/cm3 and 10.5 g/cm3, respectively. if the total volume of the jewelry is the sum of the volumes of the gold and silver that it contains, calculate the percentage of gold (by mass) in the jewelry.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:40, MathChic68
It is important to wear proper protective equipment in lab even when not actively performing experiments because accidents can affect any researcher, even one not working on an experiment. select the best answer from the choices provided
Answers: 3

Chemistry, 22.06.2019 07:30, kimberlyrios12p0ts98
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2

Chemistry, 22.06.2019 10:00, JOEFRESH10
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

Chemistry, 22.06.2019 17:30, kiaramccurty
What type of organic molecule comprises the majority of a potato?
Answers: 1
Do you know the correct answer?
Consider a piece of gold jewelry that weighs 9.85 g and has a volume of 0.675 cm3 . the jewelry cont...
Questions in other subjects:

Mathematics, 25.09.2019 03:00







Social Studies, 25.09.2019 03:00