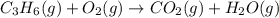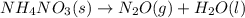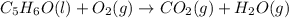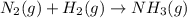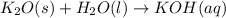Chemistry, 28.08.2019 17:20, mauricio18s
Balance the following equations, and indicate whether they are combination, decomposition, or combustion reactions.1. c3h6(g)+o2(> co2(g)+h2o(g)1a. is it a combination reaction, decomposition reaction, or combustion reaction2. nh4no3(> n2o(g)+h2o(l)2a. is it a combination reaction, decomposition reaction, or combustion reaction3. c5h6o(l)+o2(> co2(g)+h2o(g)3a. is it a combination reaction, decomposition reaction, or combustion reaction4.n2(g)+h2(> nh3(g)? 4a. is it a combination reaction, decomposition reaction, or combustion reaction5. k2o(s)+h2o(> koh(aq)5a. is it a combination reaction, decomposition reaction, or combustion reaction

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:10, hadellolo8839
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1



Chemistry, 22.06.2019 12:40, valenzueladomipay09u
How does concentration affect reaction rate
Answers: 2
Do you know the correct answer?
Balance the following equations, and indicate whether they are combination, decomposition, or combus...
Questions in other subjects:




Mathematics, 07.07.2019 20:30



English, 07.07.2019 20:30

Mathematics, 07.07.2019 20:30

History, 07.07.2019 20:30