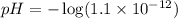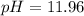Chemistry, 28.08.2019 05:30, reearamrup27
25 ml of 0.10 m aqueous acetic acid is titrated with 0.10 m naoh(aq). what is the ph after 30 ml of naoh have been added?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, kingteron5870
Ionic compounds are made of ions, and yet the overall charge of an ionic compound is neutral. why?
Answers: 1

Chemistry, 22.06.2019 01:50, lildestinyquintana
Ase your answer to this question on the information below. hydrocarbons and fissionable nuclei are among the sources used for the production of energy in the united states. a chemical reaction produces much less energy than a nuclear reaction per mole of reactant. the balanced chemical equation below represents the reaction of one molecule of a hydrocarbon with two molecules of oxygen. chemical equation: ch4 + 2o2 → co2 + 2h2o + 1.48 × 10−18 jthe nuclear equation below represents one of the many possible reactions for one fissionable nucleus. in this equation, x represents a missing product. nuclear equation: write an isotopic notation for the missing product represented by x in the nuclear equation.
Answers: 1

Do you know the correct answer?
25 ml of 0.10 m aqueous acetic acid is titrated with 0.10 m naoh(aq). what is the ph after 30 ml of...
Questions in other subjects:

Spanish, 19.02.2021 19:20

Mathematics, 19.02.2021 19:20

History, 19.02.2021 19:20

Health, 19.02.2021 19:20

Chemistry, 19.02.2021 19:20

Mathematics, 19.02.2021 19:20

Biology, 19.02.2021 19:20

Mathematics, 19.02.2021 19:20

Mathematics, 19.02.2021 19:20


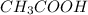 and
and 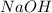 .
.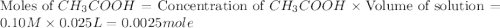
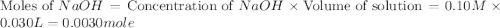
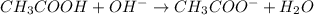
![[OH^-]=\frac{\text{Moles of }OH^-}{\text{Total volume}}](/tpl/images/0204/7530/6a358.png)
![[OH^-]=\frac{0.0005mole}{(25+30)mL}=9.09\times 10^{-6}mole/mL=9.09\times 10^{-3}M](/tpl/images/0204/7530/298a4.png)
![[H^+][OH^-]=K_w](/tpl/images/0204/7530/55f9c.png)
![[H^+]\times 9.09\times 10^{-3}=1.0\times 10^{-14}](/tpl/images/0204/7530/93bc8.png)
![[H^+]=1.1\times 10^{-12}M](/tpl/images/0204/7530/2b030.png)
![pH=-\log [H^+]](/tpl/images/0204/7530/37e81.png)