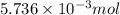Chemistry, 28.08.2019 00:30, raveransaw
How many moles of chloride ions are in 0.2550 g of aluminum chloride using alcl3 's molar mass of 133.34 g / mol: ?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 06:00, wirchakethan23
When hydrogen peroxide (h2o2) is added to potassium iodide (ki) solution, the hydrogen peroxide decomposes into water (h2o) and oxygen (o2). the chemical equation for the decomposition reaction is: 2h2o2—> 2h2o + o2. what is the role of the potassium iodide in this reaction? a. reactant. b. product. c. precipitate. d. catalyst.
Answers: 1


Chemistry, 23.06.2019 19:30, kortetsosie8813
⁉️how many kj of energy would be needed to convert 150. g of ammonia to vapor at its boiling point? ⁉️(ammonia’s heat of vaporization is 1.38 kj/g
Answers: 1
Do you know the correct answer?
How many moles of chloride ions are in 0.2550 g of aluminum chloride using alcl3 's molar mass of 13...
Questions in other subjects:




Chemistry, 15.04.2021 21:20

Mathematics, 15.04.2021 21:20


Mathematics, 15.04.2021 21:20



Mathematics, 15.04.2021 21:20


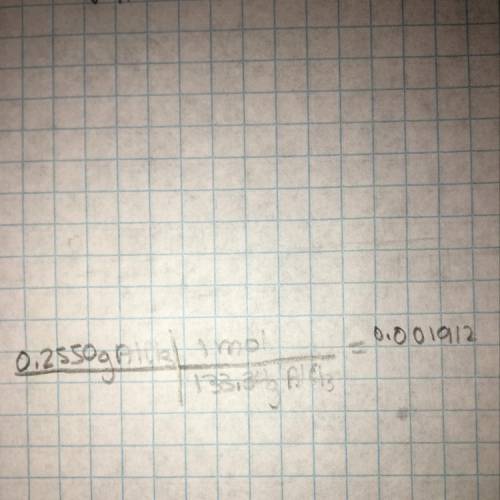
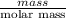
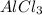 is 0.255 g and molar mass of
is 0.255 g and molar mass of 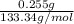
 mol
mol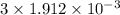 mol
mol