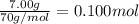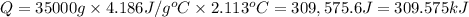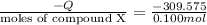Chemistry, 27.08.2019 17:10, sakurauchiha913
7.00g of compound x with molecular formula c5h10 are burned in a constant-pressure calorimeter containing 35.00kg of water at 25°c. the temperature of the water is observed to rise by 2.113°c. (you may assume all the heat released by the reaction is absorbed by the water, and none by the calorimeter itself.) calculate the standard heat of formation of compound x at 25°c. be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits. [could you show a step by step way to solve this]

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:40, gabrielolivas59
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 06:00, mapoohdoll
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1
Do you know the correct answer?
7.00g of compound x with molecular formula c5h10 are burned in a constant-pressure calorimeter conta...
Questions in other subjects:



Mathematics, 03.05.2020 12:49

Chemistry, 03.05.2020 12:49


English, 03.05.2020 12:49

History, 03.05.2020 12:49


History, 03.05.2020 12:49