
Chemistry, 27.08.2019 16:30, brandy2115
Na2co3 + hcl → co2 + h2o + naclconsider the above unbalanced equation. for this reaction, how many ml of a 2 m solution of na2co3 are required to produce 11.2 l of co2 at stp?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, alexmarche4675
Mrs. smith ordered a root beer float (vanilla ice cream + root beer). mrs. smith noticed that the three states of matter (solid, liquid, and gas) all existed simultaneously in her root beer float. a. identify each phase of matter in the root beer float. b. describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? ) c. identify one phase change you would see in a root beer float and described what causes this change.
Answers: 2

Chemistry, 22.06.2019 00:30, boonkgang6821
The clouds are grey and ground is wet. a quantitative b qualitative
Answers: 1

Chemistry, 22.06.2019 07:30, candigirl8847
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1
Do you know the correct answer?
Na2co3 + hcl → co2 + h2o + naclconsider the above unbalanced equation. for this reaction, how many m...
Questions in other subjects:

Mathematics, 16.07.2019 22:30

Computers and Technology, 16.07.2019 22:30

Biology, 16.07.2019 22:30


Mathematics, 16.07.2019 22:30

Mathematics, 16.07.2019 22:30

Health, 16.07.2019 22:30

English, 16.07.2019 22:30



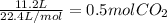
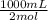 = 250 mL
= 250 mL



