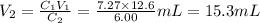Chemistry, 27.08.2019 03:10, amulets7017
Achemist must dilute 12.6ml of 7.27 m aqueous sodium nitrate (nano3) solution until the concentration falls to 6.00 m. he'll do this by adding distilled water to the solution until it reaches a certain final volume. calculate this final volume, in milliliters. be sure your answer has the correct number of significant digits.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:00, njones58emailtjcedu
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 20:00, bbyjean9974
State one important difference between a physical change and a chemical change?
Answers: 1

Chemistry, 22.06.2019 22:30, StupidFatChipmunk
What must be in balance for temperatures to remain constant?
Answers: 1

Chemistry, 23.06.2019 07:20, prettydoll19
Which statement explains which component is likely to be more powerful in explaining a scientific phenomenon? a) component c, because a theory is often passed on possibility and not certainty b) component d, because a hypothesis is often based on possibility not certainty c) component c, because the ability to explain several occurrences in the natural world is a characteristic of a hypothesis d) component d, because the ability to explain several occurrences in the natural world is a characteristic of a theory
Answers: 3
Do you know the correct answer?
Achemist must dilute 12.6ml of 7.27 m aqueous sodium nitrate (nano3) solution until the concentratio...
Questions in other subjects:



Social Studies, 12.10.2019 16:30


Chemistry, 12.10.2019 16:30

History, 12.10.2019 16:30

English, 12.10.2019 16:30

History, 12.10.2019 16:30


History, 12.10.2019 16:30


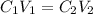
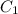 is initial concentration,
is initial concentration, 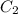 is the final concentration,
is the final concentration, 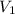 is the initial volume and
is the initial volume and 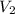 is the final volume.
is the final volume.