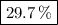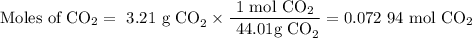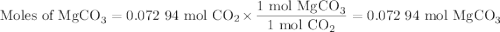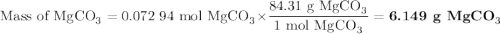Chemistry, 26.08.2019 23:30, chloiesierra29
A20.69 g sample of impure magnesium car- bonate was heated to complete decomposition according to the equation mgco3(s) → mgo(s) + co2(g) . after the reaction was complete, the solid residue (consisting of mgo and the original impurities) had a mass of 17.48 g. assum- ing that only the magnesium carbonate had decomposed, what was the percent of magne- sium carbonate in the original sample? answer in units of %.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:00, rigobertogarza2
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2


Chemistry, 22.06.2019 17:00, marsjupiter2554
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1
Do you know the correct answer?
A20.69 g sample of impure magnesium car- bonate was heated to complete decomposition according to th...
Questions in other subjects:

Mathematics, 19.03.2021 18:20

English, 19.03.2021 18:20

English, 19.03.2021 18:20

Mathematics, 19.03.2021 18:20


Biology, 19.03.2021 18:20




Spanish, 19.03.2021 18:20