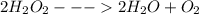Chemistry, 26.08.2019 23:30, hailiemanuel930
Acommon demonstration in chemistry courses involves adding a tiny speck of manganese(iv) oxide to a concentrated hydrogen peroxide solution. hydrogen peroxide decomposes quite spectacularly under these conditions to produce oxygen gas and steam (water vapor). manganese(iv) oxide is a catalyst for the decomposition of hydrogen peroxide and is not consumed in the reaction. what are the coefficients in the balanced chemical equation for this reaction?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, sophiapknight
Match the following items. 1. high-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. particle radiating from the nucleus of some atoms beta particle 3. negative particle identical to an electron but radiating from a decaying nucleus alpha particle
Answers: 1



Chemistry, 22.06.2019 14:00, rosetoheart2
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2
Do you know the correct answer?
Acommon demonstration in chemistry courses involves adding a tiny speck of manganese(iv) oxide to a...
Questions in other subjects:

Mathematics, 01.04.2021 08:00

Chemistry, 01.04.2021 08:00


History, 01.04.2021 08:00

Mathematics, 01.04.2021 08:00




Physics, 01.04.2021 08:00