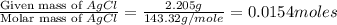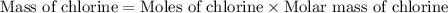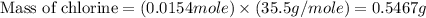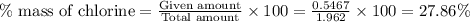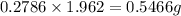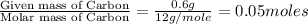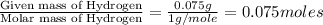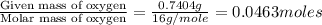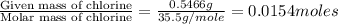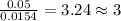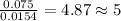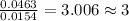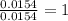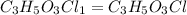Chemistry, 26.08.2019 19:30, 06laurenelizabeth
Acompound contains c, h, cl and o. combustion of 1.962 g of the compound gave 2.200 g co2 and 0.676 g h2 o. in a separate analysis 1.208 g of the compound was converted into 2.205 g agcl. the approximate molecular weight is 160. what is the empirical formula?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:50, scavalieri2421
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3

Chemistry, 22.06.2019 16:00, annsmith66
What statement goes against the kinetic theory of gases
Answers: 1

Chemistry, 22.06.2019 18:10, sangamlama
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 22.06.2019 20:30, lexibyrd120
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2
Do you know the correct answer?
Acompound contains c, h, cl and o. combustion of 1.962 g of the compound gave 2.200 g co2 and 0.676...
Questions in other subjects:




Mathematics, 02.04.2021 04:20

Mathematics, 02.04.2021 04:20

Mathematics, 02.04.2021 04:20




Mathematics, 02.04.2021 04:20


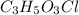
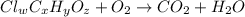
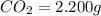
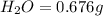
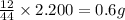 of carbon will be contained.
of carbon will be contained.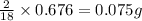 of hydrogen will be contained.
of hydrogen will be contained.