
Chemistry, 26.08.2019 18:00, juan01sebastian00
Nitrogen (n2) and hydrogen (h2) react to form ammonia (nh3). consider a mixture of six nitrogen molecules and six hydrogen molecules in a closed container. assuming the reaction goes to completion, what will the final product mixture be?
a. number of nh3 molecules
b. number of n2 molecules
c. number of h2 molecules
which of the following equations best represents this reaction?
a. 42 n2 + 6 h2 4 nh3
b. 6 n2 + 6 h2 4 nh3 + 4 n2
c. n + 3 h2 nh3
d. n2 + 3 h2 2 nh3
e. n2 + h2 nh3

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:00, JJlover1892
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 22.06.2019 23:30, billybob8514
To find the work done, the force exerted and distance moved are multiplied. a couch is moved twice before you are happy with its placement. the same force was used to move the couch both times. if more work is done the first time it is moved, what do you know about the distance it was moved? a) when more work was done, the couch was moved the same distance. b) when more work was done, the couch was moved less. c) when more work was done, the couch was moved further. d) when more work was done, the couch wasn't moved at all.
Answers: 1

Chemistry, 23.06.2019 01:00, daniel1480
Which fossil fuel is mainly used for heating and cooking? a. electricity b. coal c. petroleum d. natural gas
Answers: 2

Chemistry, 23.06.2019 01:30, jonmorton159
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1
Do you know the correct answer?
Nitrogen (n2) and hydrogen (h2) react to form ammonia (nh3). consider a mixture of six nitrogen mole...
Questions in other subjects:











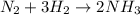
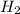 react completely with 1 molecule of
react completely with 1 molecule of 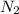 and produce 2 molecules of
and produce 2 molecules of 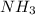 .
.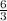 or 2 molecules of
or 2 molecules of  or 4 molecules of
or 4 molecules of 



