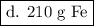Iron reacts with steam according to the reaction 3fe +4 h2o → fe3o4 + 4h2o.
how many grams of...

Chemistry, 25.08.2019 23:30, kadenreynolds5
Iron reacts with steam according to the reaction 3fe +4 h2o → fe3o4 + 4h2o.
how many grams of iron will react with 5 moles of steam to convert to iron oxide with 100% product yield?
a. 72g
b. 90g
c. 168g
d. 210g

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:30, ruleolivas
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 03:40, kellypechacekoyc1b3
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3
Do you know the correct answer?
Questions in other subjects:





Chemistry, 03.08.2019 18:20



Mathematics, 03.08.2019 18:20