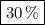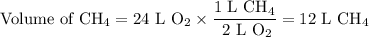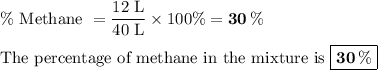Chemistry, 23.08.2019 22:30, webbhlharryteach
Forty litres of a mixture of methane and nitrogen requires 24 l of pure oxygen for complete combustion. the percentage of methane in the mixture is (a) 40 (b) 25 (c) 30 (d) 60

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:50, kelonmazon2492
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 18:30, kate3887
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1

Chemistry, 23.06.2019 01:00, akluke6059
Which statement characterizes synthetic polymers? a. they come from animals and plants. b. they are found in nature. c. they are made in a lab. d. they are components of starch.
Answers: 1
Do you know the correct answer?
Forty litres of a mixture of methane and nitrogen requires 24 l of pure oxygen for complete combusti...
Questions in other subjects:

Mathematics, 21.01.2021 01:00

Mathematics, 21.01.2021 01:00

Mathematics, 21.01.2021 01:00




Mathematics, 21.01.2021 01:00


Mathematics, 21.01.2021 01:00

Mathematics, 21.01.2021 01:00