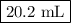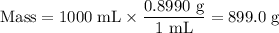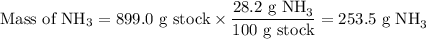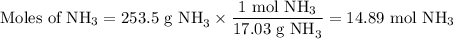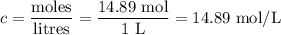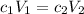Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, stephstewart1209
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 17:30, TheViperMlg23676
What causes most sediment to wash or fall into a river
Answers: 1


Chemistry, 23.06.2019 01:00, only1cache
Which is true concerning the products and reactants of photosynthesis and cellular respiration? a. the products of photosynthesis are sugars and the reactants of cellular respiration are starches. b. the products of photosynthesis are reactants in cellular respiration. c. oxygen is needed for photosynthesis and is given off in cellular respiration.
Answers: 2
Do you know the correct answer?
Astock solution is 28.2 percent ammonia (nh3) by mass, and the solution has a density of 0.8990 gram...
Questions in other subjects:


History, 25.11.2020 20:50

Health, 25.11.2020 20:50


History, 25.11.2020 20:50


Geography, 25.11.2020 20:50


English, 25.11.2020 20:50

History, 25.11.2020 20:50