

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:10, kakesheco4210
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3


Chemistry, 22.06.2019 09:20, UsedForSchool2018
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3
Do you know the correct answer?
The enthalpy of vaporization of liquid water at 100°c is 2257 kj/kg. determine the enthalpy for apor...
Questions in other subjects:


Chemistry, 12.01.2021 01:00

Mathematics, 12.01.2021 01:00

Mathematics, 12.01.2021 01:00

Chemistry, 12.01.2021 01:00



History, 12.01.2021 01:00


Spanish, 12.01.2021 01:00


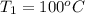 ,
, 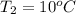
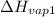 = 2257 kJ/kg,
= 2257 kJ/kg, 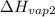 = ?
= ?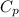 = 4.184
= 4.184 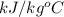
 =
= 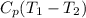
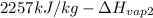 =
= 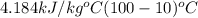
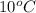 is 1880.44 kJ/kg.
is 1880.44 kJ/kg.



