
Ch4 and h2o were mixed in a 0.64 l reactor at 1800 k. steam reforming took place according to: ch4 (g) + h2o (9) co (g) + 3 h2 (9) the equilibrium constant for this reaction is k+0.28. at equilibrium, the reactor contained 0.36 mol of co, 0.081 mol of ha and 0.051 mol of ch. what is the concentration of h20 at equilibrium?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, mgavyn1052
Write a paragraph that provides examples of each stage of volcanic activity, a description of the volcano, and facts about each stage.
Answers: 1

Chemistry, 22.06.2019 14:50, wcraig1998
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1

Chemistry, 23.06.2019 01:00, bsheepicornozj0gc
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1

Chemistry, 23.06.2019 08:20, debramknoxx
At which temperature would a reaction with ah= -220 kj/mol and as=-0.05 kj/(mol-k) be spontaneous?
Answers: 2
Do you know the correct answer?
Ch4 and h2o were mixed in a 0.64 l reactor at 1800 k. steam reforming took place according to: ch4...
Questions in other subjects:


Physics, 19.02.2021 21:40



Mathematics, 19.02.2021 21:40

English, 19.02.2021 21:40

Mathematics, 19.02.2021 21:40


English, 19.02.2021 21:40




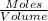
![[CO]=\frac{0.36 mol}{0.64 L}](/tpl/images/0189/2611/ede89.png)
![H_2=[H_2]=\frac{0.081 mol}{0.64 L}](/tpl/images/0189/2611/21859.png)
![CH_4=[CH_4]=\frac{0.051 mol}{0.64 L}](/tpl/images/0189/2611/7135e.png)
![H_2O=[H_2O]=?](/tpl/images/0189/2611/180f8.png)
![K_c=\frac{[CO][H_2]^3}{[CH_4][H_2O]}](/tpl/images/0189/2611/498b4.png)
![0.28=\frac{\frac{0.36 mol}{0.64 L}\times (\frac{0.081 mol}{0.64 L})^3}{\frac{0.051 mol}{0.64 L}\times [H_2O]}](/tpl/images/0189/2611/960b2.png)
![[H_2O]=0.05110 mol/L](/tpl/images/0189/2611/997c7.png)





