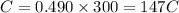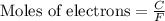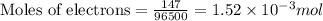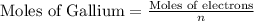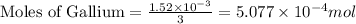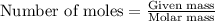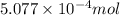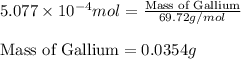Chemistry, 22.08.2019 23:10, student0724
Gallium is produced by the electrolysis of a solution obtained by dissolving gallium oxide in concentrated naoh(aq). calculate the amount of ga(s) that can be deposited from a ga(iii) solution by a current of 0.490 a that flows for 50.0 min.

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 21:00, cxttiemsp021
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1

Chemistry, 22.06.2019 22:30, robertss403
How many moles of kci are produced from 2.50 moles k
Answers: 1
Do you know the correct answer?
Gallium is produced by the electrolysis of a solution obtained by dissolving gallium oxide in concen...
Questions in other subjects:

SAT, 28.12.2021 02:20

Physics, 28.12.2021 02:20

Business, 28.12.2021 02:20


SAT, 28.12.2021 02:20



Mathematics, 28.12.2021 02:20

SAT, 28.12.2021 02:20


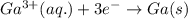
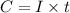
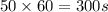 (Conversion factor: 1 min = 60 s)
(Conversion factor: 1 min = 60 s)