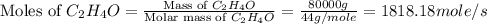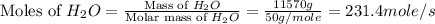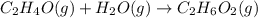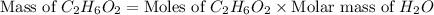Chemical reactions and stoichiometry q2. ethylene glycol (c2hs02) can be produced from the reaction of ethylene oxide (c2h40) and water as shown below. 2h40(g) t 20(g) c2h602(0) if 80 kg/s of c2h40 is mixed with 25.5 lbm/s of water, a) what is the limiting reactant? b) how much ethylene glycol is produced when the reaction is complete? c) how much ethylene glycol is produced if c2h40 is 50% pure

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, megaaan214p61pb7
Which compounds have the empirical formula ch2o? a. c2h4o2 b. c3h6o3 c. ch2o2 d. c5h10o5 e. c6h12o6
Answers: 3

Chemistry, 22.06.2019 03:40, 19thomasar
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 04:00, fantasticratz2
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2
Do you know the correct answer?
Chemical reactions and stoichiometry q2. ethylene glycol (c2hs02) can be produced from the reaction...
Questions in other subjects:





English, 29.08.2019 21:40

English, 29.08.2019 21:40


English, 29.08.2019 21:40

English, 29.08.2019 21:40

Chemistry, 29.08.2019 21:40


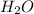
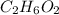 is, 11570 grams
is, 11570 grams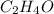 = 80 kg/s = 80000 g/s
= 80 kg/s = 80000 g/s