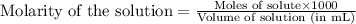Which of the following is true regarding the concentration of solutions?
a. percent solution...

Chemistry, 22.08.2019 20:30, ImGoodAtLife7797
Which of the following is true regarding the concentration of solutions?
a. percent solutions are parts per 1000 parts.
b. molarity is one mole of solute per 1000 ml of solution.
c. to calculate molarity, one must know the atomic weight of the solvent.
d. to calculate molarity, one must know the atomic number of the solute

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, sethjohnson386pbnm3x
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 15:00, hockeykid7583
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2


Chemistry, 22.06.2019 18:10, ellemarshall13
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1
Do you know the correct answer?
Questions in other subjects:




Mathematics, 30.10.2021 02:00

History, 30.10.2021 02:00

Mathematics, 30.10.2021 02:00


Mathematics, 30.10.2021 02:00


Mathematics, 30.10.2021 02:00