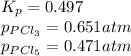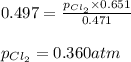Chemistry, 22.08.2019 19:20, wafflewarriormg
The equilibrium constant, kp, for the following reaction is 0.497 at 500k. pcl5(g) pcl3(g) + cl2(g)if an equilibrium mixture of the three gases in a 18.4 l container at 500k contains pcl5 at a pressure of 0.471 atm and pcl3 at a pressure of 0.651 atm, the equilibrium partial pressure of cl2 is atm.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, breannaking9734
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 18:50, emily9656
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 22.06.2019 23:30, treylartigue
The appropriate concentration for an iodine sanitizer is
Answers: 1

Chemistry, 23.06.2019 10:00, ANONYMUSNESS8670
Lord kelvin described the concept of absolute zero temperature and the laws relating to the change of thermal energy during chemical reactions what type of chemist would he be considered today
Answers: 1
Do you know the correct answer?
The equilibrium constant, kp, for the following reaction is 0.497 at 500k. pcl5(g) pcl3(g) + cl2(g)i...
Questions in other subjects:

History, 20.09.2020 03:01


Mathematics, 20.09.2020 03:01

Spanish, 20.09.2020 03:01

Chemistry, 20.09.2020 03:01

Mathematics, 20.09.2020 03:01


SAT, 20.09.2020 03:01



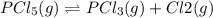
 for above reaction follows:
for above reaction follows: