Chemistry, 22.08.2019 16:30, gujacksongu6
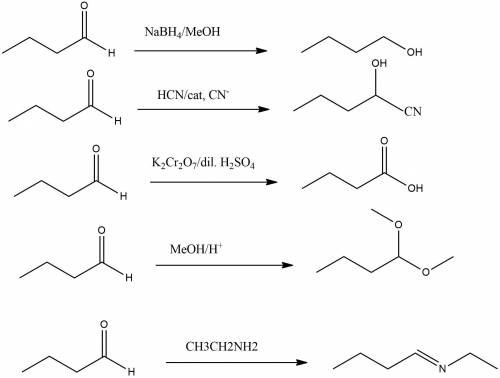
Give the products of the reaction of butanal, with: (a) sodium borohydride, in methanol (b) hydrogen cyanide, with catalytic cyanide (c) potassium dichromate in dilute sulfuric acid; (d) methanol, with a trace of acid catalyst; (e) ethylamine (ch3ch2nh2)

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:30, luhmimi17
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 11:40, tatemelliott
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3
Do you know the correct answer?
Give the products of the reaction of butanal, with: (a) sodium borohydride, in methanol (b) hydroge...
Questions in other subjects:

Mathematics, 20.10.2020 21:01

Social Studies, 20.10.2020 21:01


World Languages, 20.10.2020 21:01

Mathematics, 20.10.2020 21:01






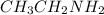 to produce imine.
to produce imine.