
Chemistry, 22.08.2019 16:10, achsahjosey
An experiment is carried out to produce hydrogen gas. hydrochloric acid is poured into a glass, and magnesium is added to the acid. the reaction produces a soluble salt and hydrogen gas. 2.1 write a balanced equation for the reaction. 2.2 give the name of the soluble salt. 2.3 has a physical or chemical change taken place? 2.4 2 moles of hydrogen is produced. how many moles of acid reacted?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, chameleonsarelife
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3

Chemistry, 22.06.2019 09:00, alydiale584
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1

Chemistry, 22.06.2019 09:00, stelllllllllllllllla
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 12:40, whitethunder05
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2
Do you know the correct answer?
An experiment is carried out to produce hydrogen gas. hydrochloric acid is poured into a glass, and...
Questions in other subjects:









Mathematics, 27.08.2020 02:01

Mathematics, 27.08.2020 02:01


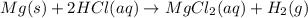
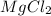
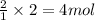 of HCl
of HCl



