Chemistry, 21.08.2019 18:20, allieballey0727
Consider a tank having one inlet molar flowrate qi and one outlet molar flowrate q. it is assumed that the pressure of the gas in the tank is p, the volume of the tank is v and temperature is t. the ideal gas law can be applied. if the volume v and temperature t are constant, find how the pressure varies with time.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, latoyatuggle23
Endeleev saw trends in the physical and chemical properties of elements when he organized them by
Answers: 2

Chemistry, 22.06.2019 04:40, shanicar33500
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 22.06.2019 08:30, lpssprinklezlps
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 22.06.2019 11:30, charles8527
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2
Do you know the correct answer?
Consider a tank having one inlet molar flowrate qi and one outlet molar flowrate q. it is assumed th...
Questions in other subjects:




Mathematics, 23.04.2021 16:10





Mathematics, 23.04.2021 16:10


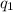

 =
= 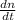 ....... (1)
....... (1)
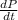 =
= 
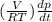
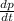 =
=